Nuclear measurement by calorimetry: application to the characterization of nuclear waste and materials

CALORIMETRY CHARACTERIZATION
Different existing mature techniques can be envisaged for characterizing the amount of nuclear material such as plutonium and other safety relevant or declarable radionuclides present in waste, in up to 200 L drums.
Calorimetry, that measures the heat released by the nuclear decay of any radioactive material, is among these techniques. It benefits from :
- being a non-destructive assay (NDA)
- being independant of the waste matrix
- being very accurate
It is used especially for tritium, plutonium and americium quantification based on the knowledge of the isotopy.
APPLICATIONS OF CALORIMETRY
- FOR INVENTORY
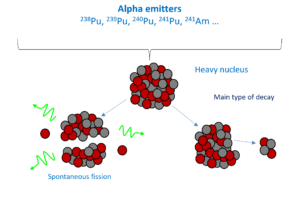
- Alpha emitters if associated with gamma ray spectrometry
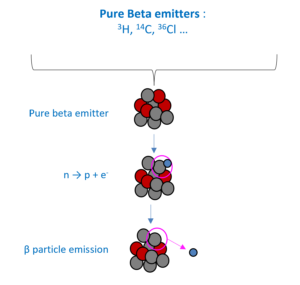
- Pure Beta emitters
- FOR WASTE CHARACTERIZATION,
- to establish the existence of radionuclides
- to look to hidden/shielded gamma or neutron source
- to look to hidden alpha sources
ADVANTAGES OF CALORIMETRY ASSAY
- NDA method
- (hidden) heat sources cannot be missed
- Independent of type of matrix and density
- Independent from the source distribution and localization inside the drum
- Independent of the gamma and neutron background
- Simple to set up and to use
- Very accurate (less than few % of uncertainty)
- Measurement possible in solid, liquid, gaseous phases
WHAT IS NUCLEAR DECAY?
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is an atom that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways:
- emitted from the nucleus as gamma radiation;
- transferred to one of its electrons to release it as a conversion electron ;
- or used to create and emit a new particle (alpha particle or beta particle) from the nucleus.
During those processes, the radionuclide is said to undergo radioactive decay. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay.
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay.
Nuclear decay leads to the released of heat. The thermal power emitted is directly related to the quantity of the radioactive material. This is the base of nuclear calorimetry measurement, where heat sensors measure the thermal power generated by a drum, which allows inferring the quantity of the radioactive material in it.
Alpha particles, also called alpha ray or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus.
A gamma ray, or gamma radiation, is a penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves and so imparts the highest photon energy.
Alpha radiation consists of helium nuclei and is readily stopped by a sheet of paper. Beta radiation, consisting of electrons or positrons, is stopped by thin aluminum plate, but gamma radiation requires shielding by dense material such as lead, or concrete.
The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly greater than that of a proton.
DEDICATED PRODUCTS LINE
HEAT-CHECK solutions can determine the mass of nuclear material in a container. They use calorimetry, by measuring the heat released during the decay of radioactive materials. It is the ideal addition to gamma spectrometry:
- Calorimetry measures a global quantity of material, but when it is associated with a gamma spectrum, it can indicate the mass of each isotope in the container
- Unlike gamma spectrometry, its measurement results are not affected by the material’s matrix, container, or conditioning
Calorimetry is considered as the most reliable technique to characterize pure beta emitters like tritium. Calorimeters from the HEAT-CHECK line provide more accurate and precise results. They use our proprietary technologies for thermal power (or heat flow) measurements, that comply with international standards like ASTM C1458.



